In order to be a competent histologist that has the ability to troubleshoot processing and staining issues in the histology laboratory, you must have a basic understanding of how atoms and molecules bind together and react.
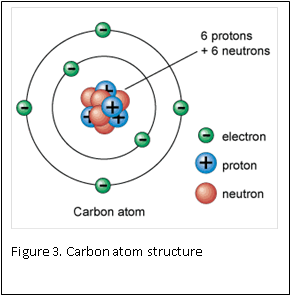
Atoms are the smallest building blocks of molecules. Atoms are composed of a nucleus, which contains protons and neutron, that are surrounded by electrons. Protons have a positive charge, electrons have a negative charge, and neutrons are neutral – they have zero charge. One of the basic rules in chemistry is: opposites attract. This rule is the basis for atoms staying together: the negative electrons are attracted to the positive protons located inside the nucleus.
Back when I was in school (when the world was considered to be flat), atomic structure was likened to the planets orbiting the son: the nucleus was the sun, and the electrons orbited around it. Today, the facts support the idea of an “electron cloud” around each nucleus. Atoms may “share” their electron cloud with the same, or other different atoms, which binds them into molecules.
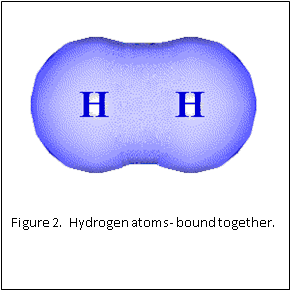
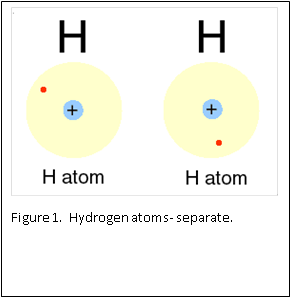
Figure 1 and 2 show how two separate hydrogen atoms bind together to make a hydrogen molecule. Hydrogen atoms are very stable when sharing their one electron, and this principle extends to all atoms: they “like” their first binding level of to be filled with two electrons. A carbon atom has a second, outer level that wants to be filled with eight electrons. It has four, so is always “looking” for four other atoms to share electrons with (Figure 3).
Histology involves using formaldehyde to chemically “fix” dynamic, living tissue into a static “snapshot” of cellular activity. The cells in your body are currently metabolizing energy sources, and performing chemical reactions to ensure that all of your bodily functions continue, and you stay alive. When tissue is removed from the body (i.e. surgery or biopsy), the cells no longer receive oxygen from the blood, and the cells begin to die, and autolyze (i.e. break down). The fixation process “fixes” the tissue, and stops the autolysis process, thereby preserving the cellular structure and tissue architecture, for subsequent processing into a paraffin block.
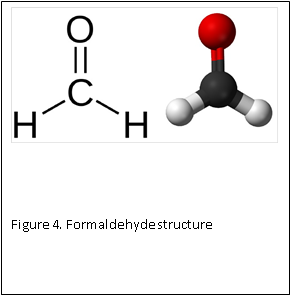
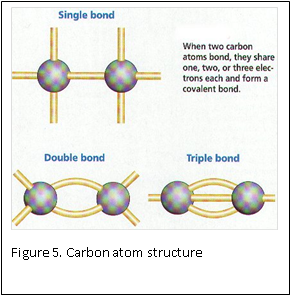
At the molecular level, formaldehyde is a simple molecule, consisting of one carbon atom joined to two hydrogen atoms with a single bond, and one oxygen atom with a double bond (Figure 4) . Carbon is stable when it forms a total of four bonds. A double bond contains a lot of energy – similar to compressing a spring. The bond wants to “spring apart” to release the energy. It does this by “springing apart’ the double bond, to provide two single bonds, which immediately bind two other molecules. This is what is meant by the term “cross linking” fixation, as relates to formaldehyde. The formaldehyde molecule cross links molecules within the protein structure of the cells (Figure 5).
Why do we need to know this?
Because living tissues are made up primarily of carbon, hydrogen and oxygen, and these are the molecules of biochemistry. Histologists need to know the chemistry of fixation, processing and staining.
Once the tissue is fixed in formalin, the proteins within are cross linked and stabilized. The tissue is in a solution of 4% formaldehyde in 96% water – similar to the natural water content of the human body. In routine histology, the final goal is to embed the tissue in a paraffin wax block. Water and wax do not mix. In order to be able to infiltrate the tissue with wax, and embed it in a paraffin block, the water has to be removed; the tissue must be dehydrated.
Dehydration is usually accomplished by using a graded series of alcohols to remove the water, and replace with 100% alcohol. Alcohol and wax do not mix. Therefore, histologists can use an “intermediate substance”, to bridge the gap between alcohol and wax. For most laboratories, this substance is xylene – although there are now substitutes that can be used.
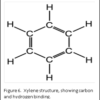
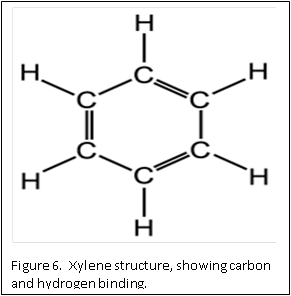
The molecular structure of xylene is shown in Figure 6. You can see that it is a “hybrid” molecule. The center is a “ring’ of carbon atoms, with alternating single and double bonds. The exterior is made up of single bonds to hydrogen. This unique structure allows xylene to mix with both alcohol and paraffin. This brings us to the second rule of chemistry: “like dissolves like”. The middle ring of xylene is described as “organic”, which is like the organic ring structure of paraffin. The exterior is a straight chain “inorganic” structure, which is like the structure of alcohol.
The principles above form the basis of understanding the chemical basis of fixation and processing of tissues in histology. Once you understand them, you can troubleshoot fixation and processing issues that will occur in your laboratory.
REFERENCES:
- Theory and Practice of Histological Techniques. Chapter 10. JD Bancroft, A Stevens ed. Churchill Livingstone, NY. Fourth edition. 1996
- Theory and Practice of Histotechnology. Chapter 9. DC Sheehan, BB Hrapchak. CV Mosby Company, St. Louis. First edition. 1980.
- CM Chapman. Histology Study Group. Presented at Region I meeting, hosted by MaSH. 2014.