The first four blogs of the troubleshooting series focused on being proactive with regard to the prevention of sub-optimal events in the histology laboratory. Unfortunately, we are not able to predict every single potential issue that may cause a sub-optimal event in the laboratory. As a result, another strategy is required. This next series of blogs explains the chemistry and theory of fixation and processing. Learning these concepts will form a basis of knowledge that will allow the histologist to troubleshoot sub-optimal events that may occur in the histology laboratory during these phases of specimen preparation.
Living tissues are made up primarily of carbon, hydrogen and oxygen and are known as the elements of biochemistry. Histologists need to know the chemistry of fixation, processing and staining. Histology involves using formaldehyde to chemically “fix” dynamic, living tissue into a static “snapshot” of cellular activity. The cells in your body are currently metabolizing energy sources and performing chemical reactions to ensure that all of your bodily functions continue, and you stay alive. When tissue is removed from the body (i.e. surgery or biopsy), the cells no longer receive oxygen from the blood, and the cells begin to die, and autolyze (i.e. break down). The fixation process “fixes” the tissue, and stops the autolysis process, thereby preserving the cellular structure and tissue architecture, for subsequent processing into a paraffin block.
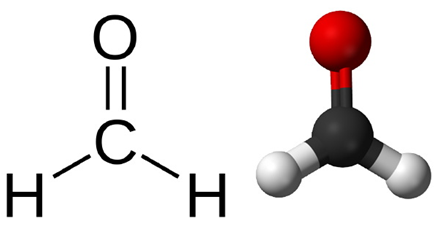
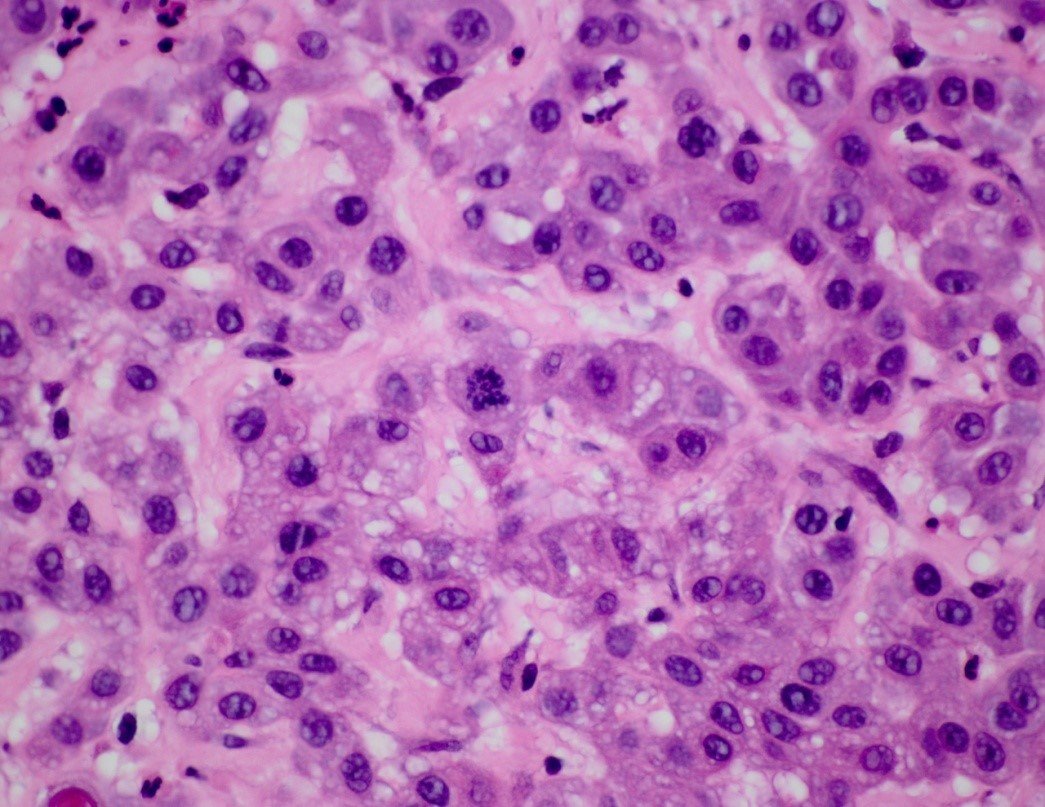
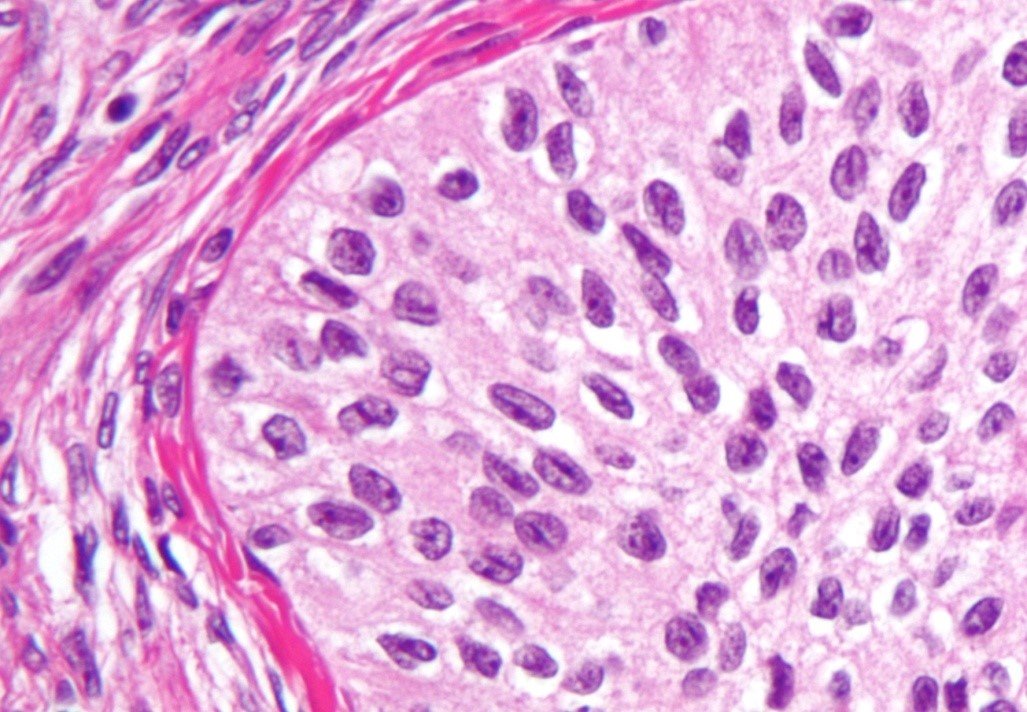
At the molecular level, formaldehyde is a simple molecule, consisting of one carbon atom joined to two hydrogen atoms with a single bond, and one oxygen atom with a double bond (Figure 1A). Carbon is stable when it forms a total of four bonds. A double bond contains a lot of energy – similar to compressing a spring. The bond wants to “spring apart” to release the energy. It does this by “springing apart” the double bond, to provide two single bonds, which immediately bind two other molecules. This is what is meant by the term “cross linking” fixation, as it relates to formaldehyde. The formaldehyde molecule cross links molecules within the protein structure of the cells. Optimal fixation is the basis of optimal processing and results in an optimal microscope slide (Figure 2A). Suboptimal fixation cannot be remedied after the slide has been made (Figure 2B). Even though there are procedures for “running back” specimens to formalin and then reprocessing them, the result will always remain sub-optimal. Therefore, it is of paramount importance to ensure that specimens are completely fixed prior to processing.
Once the tissue is fixed in formalin, the proteins within are cross linked and stabilized. The tissue is in a solution of 4% formaldehyde in 96% water – similar to the natural water content of the human body. In routine histology, the goal is to embed the tissue in a paraffin wax block. Water and wax do not mix. To be able to infiltrate the tissue with wax, and embed it in a paraffin block, the water must be removed; the tissue must be dehydrated.
Dehydration is usually accomplished by using a graded series of alcohols to remove the water and replace it with 100% alcohol. Alcohol and wax do not mix. Therefore, histologists can use an “intermediate substance”, to bridge the gap between alcohol and wax. For most laboratories, this substance is xylene – although now there are xylene-substitutes that can be used as well.
Learn more about automated tissue processors
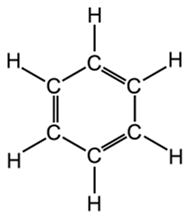
The molecular structure of xylene is shown in Figure 1B. You can see that it is a “hybrid” molecule. The center is a “ring’ of carbon atoms, with alternating single and double bonds. The exterior is made up of single bonds to hydrogen. This unique structure allows xylene to mix with both alcohol and paraffin. This brings us to the first rule of chemistry: “like dissolves like”. The middle ring of xylene is described as “organic”, which is like the organic ring structure of paraffin. The exterior is a straight chain “inorganic” structure, which is like the structure of alcohol.
The principles above form the basis of understanding the chemical basis of fixation and processing of tissues in histology. Once you understand them, you can troubleshoot fixation and processing issues that will occur in your laboratory, as we will see in the next blog.
References:
Chapman, C.M. (2017). The Histology Handbook: Amazon CreateSpace Independent Publishing Platform
Chapman, C.M. and Dimenstein, I.B. (2016). Dermatopathology Laboratory Techniques. Amazon CreateSpace Independent Publishing Platform
Chapman, C.M. (2018). Troubleshooting in the Histology Laboratory. Submitted to J Histotechnol





