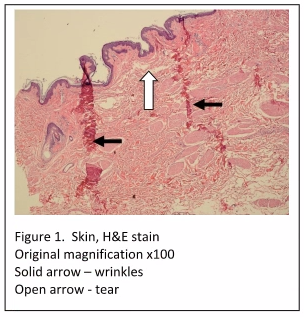
The most noticeable tissue processing artifacts are the wrinkles and tears in the tissue sections which are evident even at low power (Figure 1). Incomplete fixation would not cause such artifacts, as the cellular histology is acceptable (i.e. nuclear and cytoplasmic staining is within quality control limits). Improper embedding would also not be the cause of these artefacts. When skin specimens are mis-embedded, usually tearing and wrinkles would be localized within the epidermis, and within the dermal-epidermal junction.
The most likely cause of the artifacts seen in “Figure 1” is due to incomplete dehydration, resulting in incomplete paraffin infiltration. When tissue is not completely dehydrated, excess water is left in the tissue. If xylene is used as the clearing agent, it can dissolve up to 2% water – but no more. Also, if xylene substitutes are used, they are completely intolerant of any residual water. If water is present in the tissue after dehydration and clearing, paraffin cannot infiltrate the tissue completely. Without complete paraffin infiltration, the tissue can tear and fold during microtomy. If this affects all tissues in the run, the tissues may have to be reprocessed by melting down the blocks and placing the tissues back into the cassettes, with new cassette tops. The cassettes can now be run back through changes of xylene and 100% alcohol. After rinsing in 95% alcohol, the tissues can be put back into the tissue processor to begin processing at the formalin step. Finally, they can be run back through xylene and into paraffin. This procedure should remedy the problem.
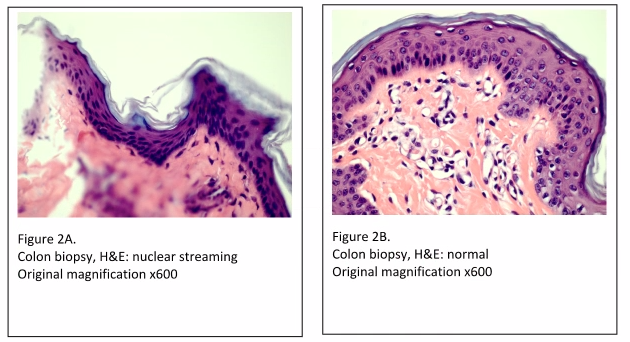
Another cause of tissue processing artifacts is referred to as “nuclear streaming” (Figures 2A and 2B). Nuclear streaming is a result of three possible circumstances; incomplete dehydration, too rapid dehydration, or processing solutions that are overused and in need of changing out. When water in the tissue moves out too quickly, resulting in “squeezing” the cell, it leaves a final appearance of the nuclei in a “streaming” configuration.
Other questions to ask when experiencing tissue artefacts are:
Did the tissue dry out during transport? If so, the client should be contacted to see if they used an empty specimen bottle, with no formalin in it. Also, the specimen bottle should be inspected to see if it was cracked and the formalin leaked out.
Are the tissue processor reagents clean / exhausted? The tissue processor maintenance logs should be checked to see when the last time the reagents were changed out. A hydrometer can be used to determine the actual percentages of the alcohol reagents.
What about the tissue processor reagent bottles? Did the tissue processor reagents get swapped? The hydrometer can be used to determine the answer. Also, do all of the reagent bottles contain fluids that measure up to the “fill” mark? There must be enough reagent in the bottles to fill the processor retort.
If you are wrapping small tissues, are the reagents getting full penetration into and through the tissue? There should be only one layer of paper surrounding the tissue, not several layers.
If you are using sponges, are reagents carrying over? Sponges are notorious for getting saturated with reagent, and then holding onto it. If enough of the reagent carries over into the next reagent bottle, it can change the concentration and/or contaminate the subsequent reagent.
If you are using biopsy cassettes, are the holes too small, which may cause what’s called “air lock”? Some biopsy cassettes have micro-holes in them. You must be sure that the holes are not so small that they prevent the proper movement of reagents through them.
So, when you see tissue artifacts on your slides, troubleshooting using the above techniques involving theory, chemistry, and considering the physics of the consumable products being used, you should be able to resolve most issues when they occur in your laboratory.
References:
- Chapman, C.M. (2017). The Histology Handbook: Amazon CreateSpace Independent Publishing Platform
- Chapman, C.M. and Dimenstein, I.B. (2016). Dermatopathology Laboratory Techniques. Amazon CreateSpace Independent Publishing Platform
- Chapman, C.M. (2018). Troubleshooting in the Histology Laboratory. Submitted to J Histotechnol


